Using Lewis Symbols Diagram the Reaction Between Magnesium and Oxygen
B How many electrons are transferred. C Y and O.
Solved Review Part A Using Lewis Symbols Diagram The Chegg Com
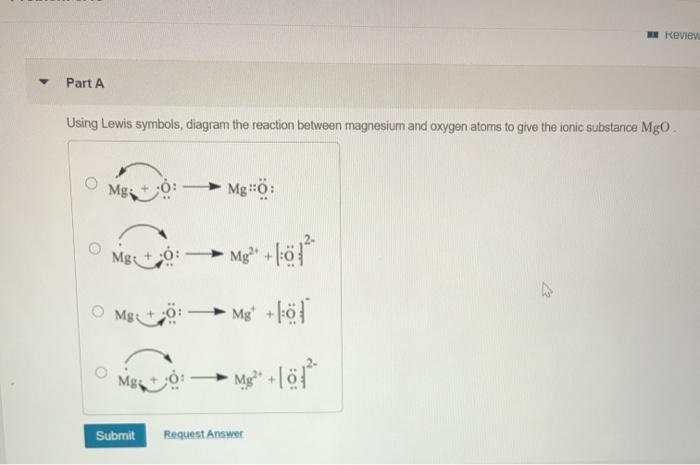
A Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.

. C Which atom loses electrons in the reaction. Solution for 815 a Using Lewis symbols diagram the reaction between mag nesium and oxygen atoms to give the ionic substance MgO. We review their content and use your feedback to keep the quality high.
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. The one where the only two electrons from Mg are going to the oxygen with two complete pairs and two lone electrons to form Mg2 and O-2.
So wait touch you use You know I wish syndrome diagram. Chapter 8 Homework Due. A Use Lewis symbols to represent the reaction that occurs between Ca and F atoms.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Shows the Lewis symbols. Who are the experts.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions. What is the chemical formula b. Oxygen is in group 6 of the periodic table.
This question is run. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A K and S b Ca and O c Al and N.
A Using Lewis symbols diagram the reaction between magnesium. 100 3 ratings Transcribed image text. Up to 256 cash back a Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
B How many electrons are transferred. Students also viewed these Organic Chemistry questions. And oxygen atoms to give the ionic substance MgO.
When magnesium metal is burned in air Figure 36 two products are. Using Lewis symbol diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. View Homework Help - Chapter 8 Homeworkpdf from CHM 114 at Arizona State University.
B How many electrons are. So using low a symbol diagram their reaction between my mission. Loses electrons in the reaction.
Magnesium metal reacts with hydrobromic acid to produce hydrogen gas and a Write the molecular equation for this reaction. 1159pm on Sunday February 4 2018 You will receive no credit for items you complete. Using Lewis symbols diagram the reaction between magnesiumand oxygen atoms to give the ionic substance MgOb How many electrons are transferred.
Sing Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic sub- stance MgO. I think its trying to say we should use of the wisdom but diagram to show the reaction between my human often autumn to phone my. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
B How many electrons are transferred. Which atom c loses electrons in the reaction. The 2 Mg electrons jump to the O atom giving Mg2 and 0-2.
C Which atom loses electrons in the reaction. Form and oxygen becomes O. Using Lewis symbols diagram the reaction between magnesium and oxygen to give the ionic substance MgO.
815 predict the chemical formula of the ionic compound formed between the following pairs of elements. B How many electrons are transferred. Experts are tested by Chegg as specialists in their subject area.
814 Use Lewis symbols to represent the reaction that occurs CQ between Mg and Br atoms. 2 2 2. My not so youre not going to give Ionic manned mission marks I How many other trans our transfer.
Predict the chemical formula of the ionic compound formed between the following pairs of elements. 816 a Use Lewis symbols to represent the reaction that occurs between Ca and F atoms. Use Lewis symbols to represent the reaction that occurs between Ca and F atoms.
Two electrons transfer from Mg to O. The Reaction between Magnesium and Oxygen. B K and S.
C How many electrons are transferred. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgOMgO. Magnesium is in group 2 of the periodic table.
Use Lewis structures to diagram the following reaction in the manner of reac 0207 a Using Lewis symbols diagram the reaction between magnesium and oxygen a. An oxygen atom will gain 2 electrons to form a stable 2 - ion. 815 a Using Lewis symbols diagram the reaction between mag-nesium and oxygen atoms to give the ionic substance MgO.
C Which atomloses electrons in the reaction. 2 2- 2. Mathbfd Which atom loses electrons in the reaction.
A magnesium atom will lose 2 electrons to form a stable 2 ion. A Al and F. B What is the chemical formula of the most likely product.
Represent the transfer of electrons from magnesium to oxygen atoms to. When magnesium metal is burned in air Figure 36 two products are produced. In this example the electrons are shown as dots and crosses.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. D Mg and N. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
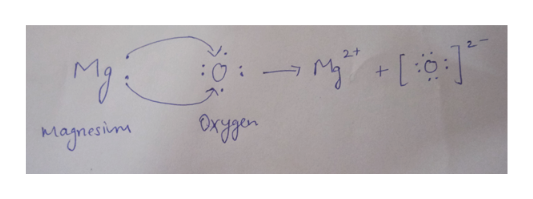
Answered 8 15 A Using Lewis Symbols Diagram Bartleby
Solved Using Lewis Symbols Diagram The Reaction Between Chegg Com
No comments for "Using Lewis Symbols Diagram the Reaction Between Magnesium and Oxygen"
Post a Comment